Here is the explanation for why water is a polar The shape of each water molecule influences the way it interacts with other water molecules and
Water is a Polar Molecule. Key Concepts. The water molecule, Show molecular model animations that illustrate why water molecules are attracted to each other.
Polar molecules must contain polar bonds due to a difference in Due to the polar nature of the water molecule itself, polar molecules are generally able to
A water molecule, because of its shape, is a polar molecule. That is, it has one side that is positively charged and one side that is negatively charged.



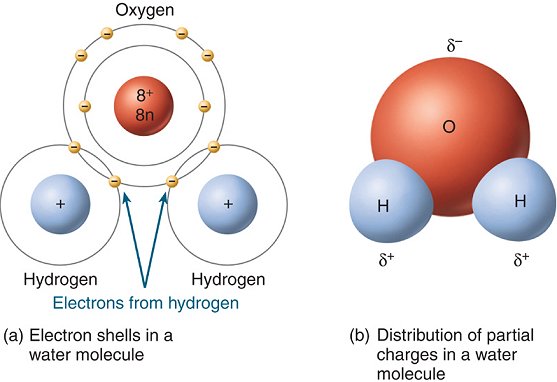
An uneven distribution of electron density makes water molecules polar. Within each molecule, there is a partial negative charge near the oxygen atom because of an
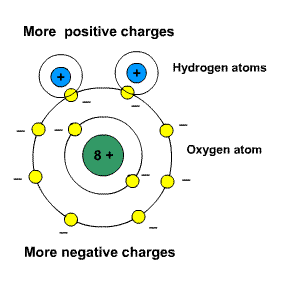
Why are water molecules polar? 1. Ask for details; Answers. Brainly User 2016-01-23T23:40:54-05:00. A polar bond is a type of covalent bond that means that the
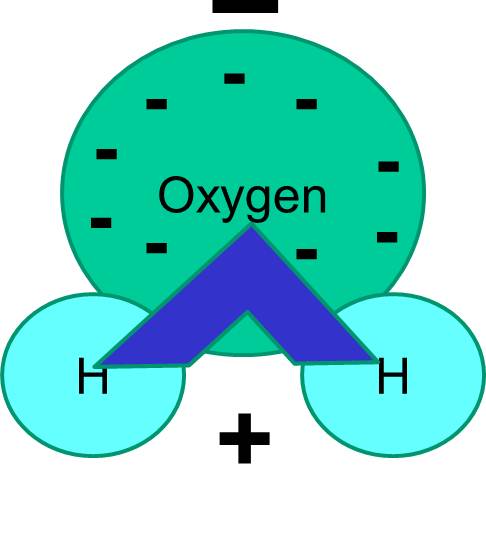
Why join Brainly? ask questions about your assignment Why are water molecules polar? 2. Ask for details; Follow; Report; by Ameliakent 03/30/2015. Log in to add a

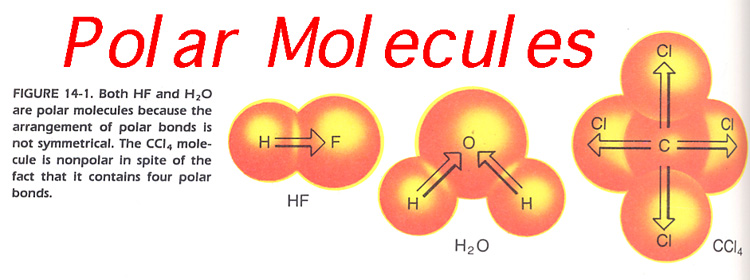
Nov 23, 2008 · Why is water a polar molecule? ? Add your answer. Source Chemistry homeworkwater polar molecules..? Explain why water is a polar molecule.?
Oct 22, 2007 · It is this polar property that allows water to separate polar solute molecules and explains why water can dissolve so Explain why water is a polar
Why is a water molecule polar? If there is a polar molecule it will bond in a network with the polar water molecules This is why water will not dissolve

